STEAM Spotlight: A Snowflake’s Journey
March 1, 2020 | by stephanie hobby
Montanans’ relationship with snow can best be described as, “It’s complicated.” When it lands on the slopes, we’re overjoyed. We’re not as happy about shoveling our driveway for the umpteenth time. By the billions, they are part of life here, but individual snowflakes are worth marveling.
It all starts with a single speck of dust, swirling somewhere around 50,000 feet or higher in the atmosphere. When conditions are prime for snowflake making, a supercooled - but not frozen - layer of moisture is nearby. As these water droplets collide with dust, pollen, or even volcanic ash, they grab on and start freezing, setting the stage for the six-sided crystals that descend to earth. The final shape they take depends on what happens on the way down.
Temperature, and to some extent, humidity, play a significant role in how the crystals form and ultimately, how the snowflake looks. As a flake makes its way to the ground, it falls through different moisture levels and temperatures, affecting how the water molecules interact and align.
Thanks to a well-established formula, we can expect the delicate, six-armed lacy dendrite snowflakes, or the plainer hexagonal plates, to fall when temperatures hover near the freezing point. As the mercury dips, snow becomes more prism or needle-like. Between 12 and -8 degrees F, you can again observe the stunning dendrite snowflakes. Below -8 degrees F, the moisture levels drop, and flakes once again resemble six-sided tubes or needles.
Keith Meier, Meteorologist In Charge at the National Weather Service Weather Forecast Office in Billings, explained that when it gets really cold, one zone in the atmosphere becomes very efficient in growing dendrite snowflakes. “There are some unique characteristics between minus 10 and 20 degrees Celsius, where supercooled water can exist; the temperature is that cold but doesn’t freeze. If the air is that chilly and has moisture in it, when ice crystals fall into that, the snowflakes grow very efficiently, producing giant, dime-sized flakes.”
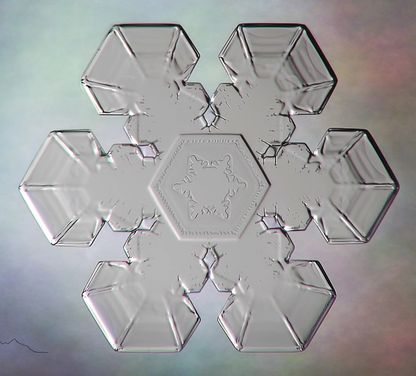
So what’s up with the six-sided symmetry? How do the different sides know how to grow to match one another? It all has to do with basic physics and chemistry. Water molecules have two hydrogen and one atom of oxygen, forming an open-sided triangle. When two water molecules join together, that sets the stage for how other molecules will arrange themselves in that structure. As the looser water molecules begin to freeze into a more rigid lattice, it becomes a bit like laying a tile floor. Once the pattern is established, others can only fit in a certain way, so as the flake sails down, it collects more water and freezes, but the molecules can only lock in through a preset pattern.
Because corners on a hexagon will snag other molecules more effectively than a flat side would, snowflakes tend to grow six "arms" as more molecules latch on. Each of the arms experiences the same atmospheric conditions, prompting the crystals to grow the same way. What's interesting is that no two snowflakes take the same path to the ground, so while two might start out similarly, they'll face different conditions as they whirl through the complex layers of the atmosphere, and could look vastly different by the time they reach your yard.
The whole process, from a speck of dust 10 miles up, to forming a crystal and landing on your driveway, usually happens in mere minutes, but can take up to an hour. Meier said you can learn a lot about what’s happening in the atmosphere by observing snowflakes; for example, if it’s turbulent, snowflakes will crash into one another on the way down, sometimes getting pushed back up toward the clouds, until they resemble tiny snowballs, called snow pellets.
Meier likens weather conditions to baking. "Every day we have kind of the same ingredients; we have air temperature, moisture, and winds, but we don't have the same weather every day," Meier said. "What makes it interesting for us is that we try to figure out the amount of various ingredients that the atmosphere has on any given day, and when you mix those together, what's the outcome? Is it rain? Is it snow? Is it big snowflakes that stack up in fluffy snow? Is it wet snow? Is it snow pellets? Ice crystals that look like sugar on your sidewalk?"
So the next time you’re out clearing the sidewalk, maybe stop and take a minute to appreciate the incredible journey those tiny flakes made. It might take your mind off the fact that you’ll be out shoveling again in an hour.
Aaand action! Observing and Preserving Snowflakes
Courtesy of Home Science Tools
When the snow starts falling, grab your coats and boots, a couple of pieces of black construction paper, and a magnifying glass or two if you have them. Catch a couple of snowflakes on your black construction paper and observe them with a magnifying glass. Compare their similarities and differences. Count how many sides or points the snowflakes have and if any appear to match.
If you have a microscope and microscope slides, try preserving snowflakes. All you need for this activity are the slides, but a microscope is a fun bonus. Prepare beforehand by placing a couple of slides in the freezer so that they won't melt the snowflakes. You’ll also need hairspray or artists' fixative. Store these items in a cold area like your refrigerator or an unheated garage. When it is time to collect and preserve snowflakes, bring out the slides, hairspray, and a couple of toothpicks.
Spray one side of the slides with hairspray. Catch the snowflakes on the sticky side of the microscope slides. Use a toothpick to gently move the snowflake to center it if needed. Place the slide with the snowflake in a cold area (such as inside a covered box or in the garage) where no more snowflakes will fall on it. Leave the slide untouched for several hours so that the hairspray can dry, and the water in the snowflake will disappear. You now have the imprint of a snowflake on a slide you can study with the naked eye or a microscope.
Originally printed in the March 2020 issue of Simply Local Magazine
Never miss an issue, check out SLM's digital editions here!